New Product 2019-nCoV(COVID-19) IgG&IgM Rapid Test Kit

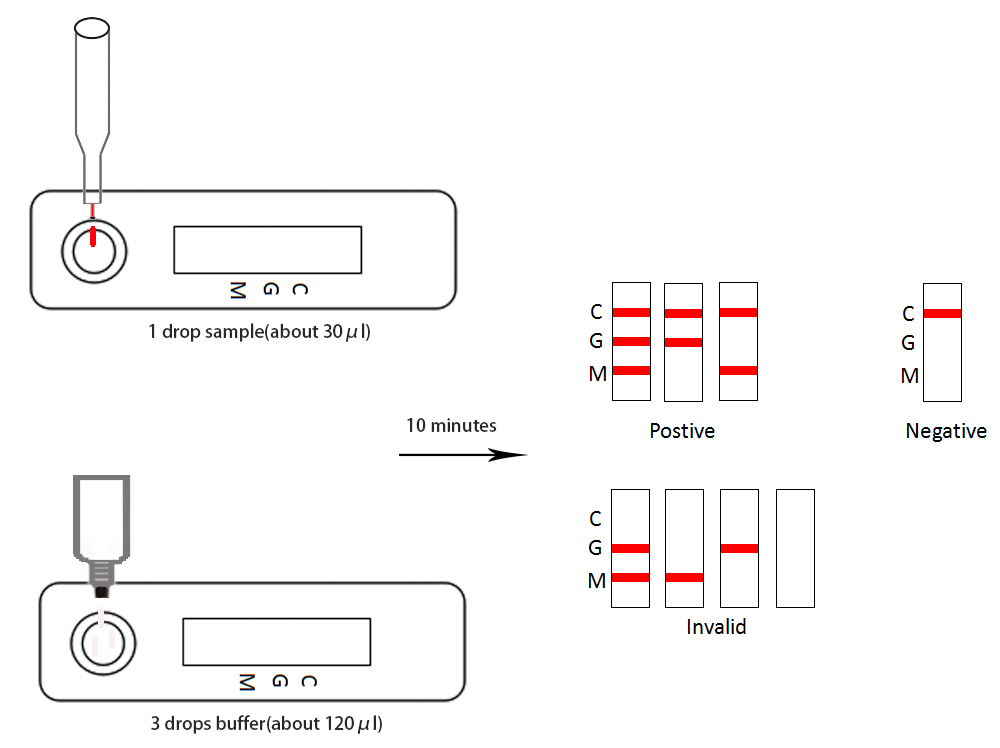
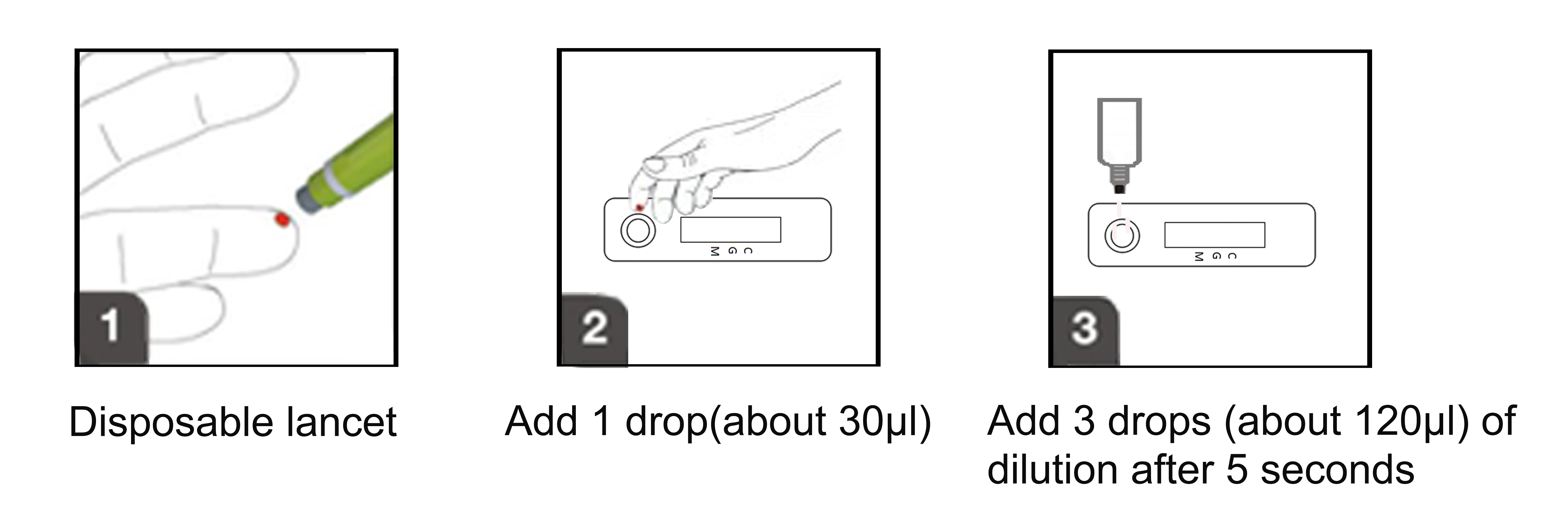
This assay use highly specific antigen-antibody responses, highly sensitive patent markers, and immunochromatography.Test card containing package is advance in glass fiber has the patent protection on the membranes of the gold tag 2019 new coronavirus antigen expression and fixed on the membrane resistance test area (G) of rat IgG antibody and test area (M) of rat IgM antibody against people and qc area (C) the sheep to test the resistance to mouse IgG, add 1 drop of sample (about 30 ul) to detect sample card with hole (S), and then add 3 drops (about 120 ul) diluent added sample hole (S), chromatography.If the sample contains 2019 new coronavirus specific IgG/IgM antibody, and the package is in advance in gold tag on a glass fiber membrane antigen of 2019 new coronavirus expressed in combination to the upper analysis, can then be fixed on the membrane test area (G) of mouse anti human IgG antibody or (M) of rat resistance IgM antibody combined with capture, red stripe, can determine the corresponding IgG and IgM.
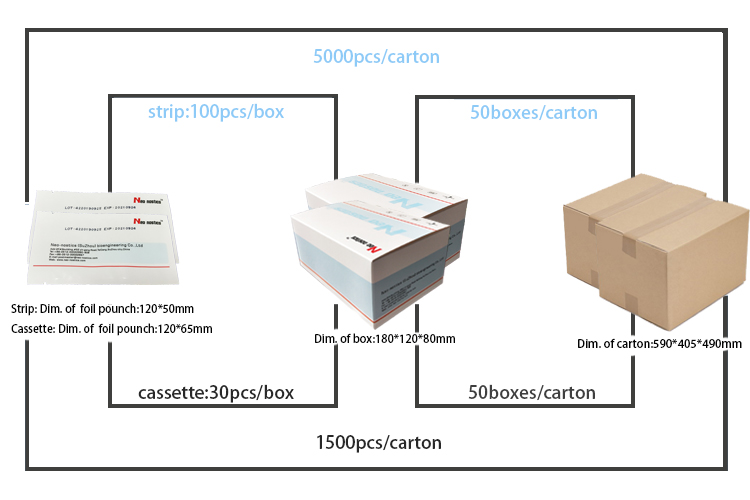
Packing & Delivery

Neo Nostics (Suzhou) Bioengineering Co., Ltd. which was established in 2015, is a professional company specialized in researching, developing, manufacturing and distributing advanced medical instruments and in-vitro medical diagnostic products. Our productsareusuallyusedforfertility tests, infectious diseases tests, drugs of abuse tests, alcohol saliva tests, urinalysis reagent strips, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests.We utilizeadvancedengineering techniques and a strong design team to help you utilize ouruniqueretail brand. We have the ability to provide quality products with simple-to-use formats that provide consistent product performance, translating it into value for you and your customers.We warmly welcome business friends from all walks of life and look forward to establishing friendly and cooperative business relations with you and achieving win-win goals.
Certificate

Exposition


