新产品SARS-CoV-2 Ag(COVID-19 Ag)快速检测试剂盒(仅供外贸使用)



COVID 19 Antigen Test Kit (Colloidal Gold Method)
Instructions for Use
For professional use
[Product name]
COVID 19 Antigen Test Kit (Colloidal Gold Method)
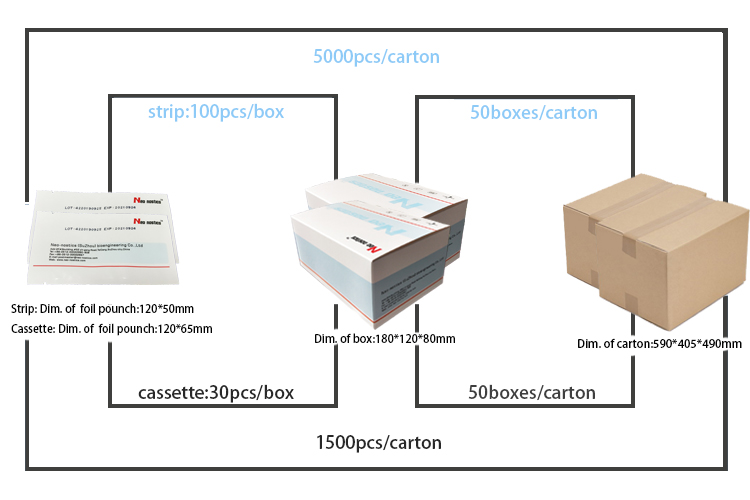
[Packaging specification]
Test cassette (single test packaging):
1 tests/box; 5 tests/box;10 tests/box; 15 tests/box.
[Intended use]
This reagent is used for in vitro qualitative test of COVID 19 antigen N protein in human Nasal /Nasopharyngeal and oropharyngeal samples. Synthetic spinous protein and genetic variants of UK cannot be detected
It is only used as a supplementary test indicator for suspected cases with negative result of COVID 19 test or combined with the 2019-nCov IgG / IgM antibody test kit for the auxiliary diagnosis of suspected cases. It cannot be used as a basis for confirmation or diagnosis of pneumonia caused by COVID 19 infection. A positive test result needs further confirmation, and a negative test result cannot exclude the possibility of infection.
The 2019 novel coronavirus is also known as COVID 19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the COVID 19 are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
[Test principle]
COVID 19 antigen test kit (colloidal gold method) was prepared by colloidal gold solid-phase immunochromatography. A monoclonal antibody COVID 19 N protein was pre immobilized on the cellulose nitrate membrane. The monoclonal antibody against the COVID 19 N protein was labeled on colloidal gold on the glass fiber membrane. The double antibody sandwich immunoassay was used to detect the N protein of the COVID 19. The kit used for in vitro qualitative auxiliary test of COVID 19 antigen samples.
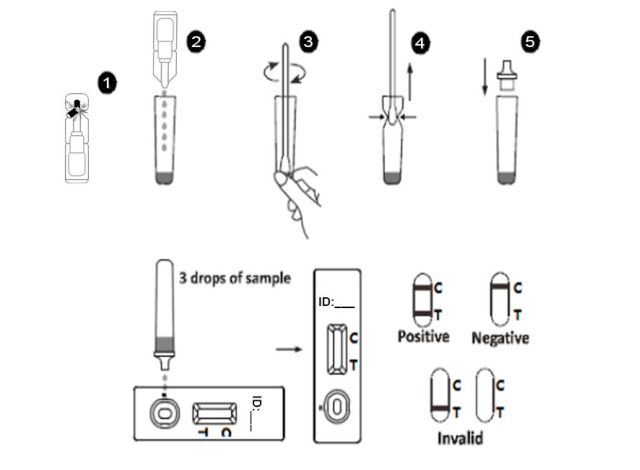
When testing, add 3 drops of pretreated sample (about 90ul) into the sample hole (s) of the test card. If the COVID 19 antigen is contained in the sample, COVID 19 antigens in the sample can form colloidal gold complexes with colloidal gold labelled antibodies. The composite is moved forward along the test strip along with the test strip and captured at the test line location (T) of nitrocellulose membrane. The excess unconjugated colloidal gold labeled antibody continued to move forward to the position of the quality control line, and was captured by the solid-phase antibody of the quality control line. In this way, a purple red band visible to the naked eye is formed at the test line position (T) and quality control line position (C), indicating a positive result. If the sample does not contain COVID 19 antigen or its content is lower than LOD, it will not show color at the test line position (T), and only purple red band will be displayed at the quality control line position (C), indicating a negative result. If there is no purplish red band at quality control line (C), it is determined that the result is invalid regardless of whether there is a purple red band at the position (T) of the test line, and it needs to be determined again.
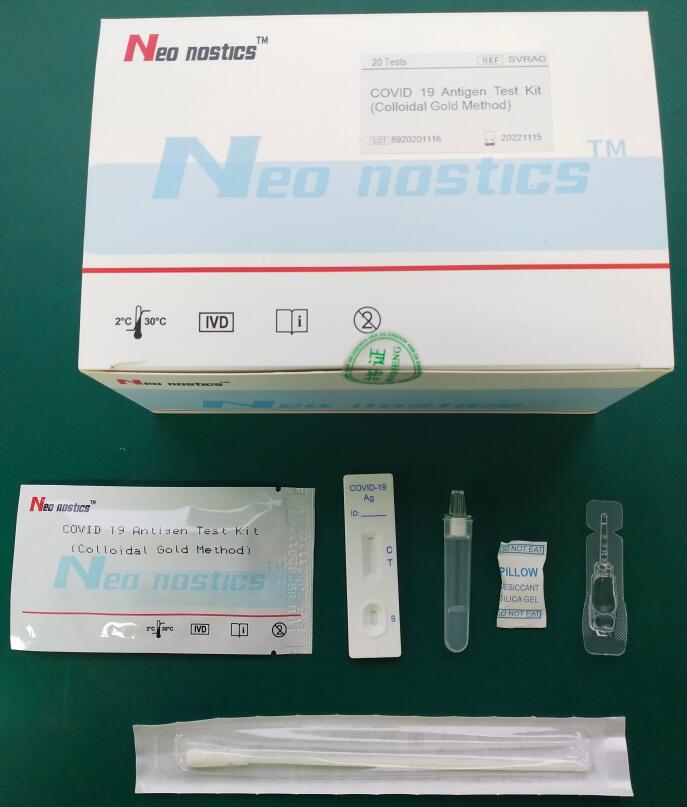
[ Components]
1.Test cassette 2. Swabs
3.Extraction tube 4.Buffer tube(600ul/bottle)
5.Instruction for Use
[Storage conditions and shelf life]
The original packaging should be stored in a dark and dry place at 2°C~30°C, and should not be frozen. The shelf life of the product is 24 months. The reagent should be used as soon as possible within 1 hour after the aluminum foil bag is opened.
Date of production: see the product label.
Date of expiration: see the product label.
[Sample collection and processing]
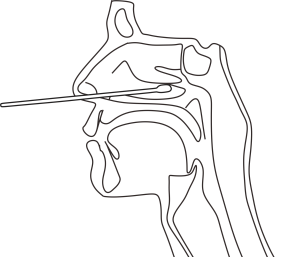
Please use the swab provided in this product for sampling. Before collecting nasal/nasopharyngeal/oropharyngeal secretions, Rotate the buffer tube provided in the test kit, add the buffer drop into the extraction tube, and then start sampling.
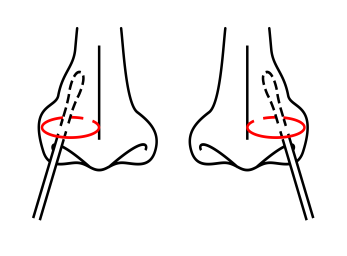
Note: When collecting the nasal secretion,gently insert the swab into the nostril 2.5-3cm away, and rotate the swab several times in a round way on the inner wall of the nostril to ensure that as many cells and mucus as possible are collected. Repeat the same process with the same swab in the other nostril.
When collecting the nasopharyngeal secretion, gently insert the swab into the most secreting part of the nostril and push it toward the nasal cavity until the turbinate bone. Rotate the swab several times in a round way on the inner wall of the nostril to ensure that as many cells and mucus as possible are collected.
When collecting the oropharyngeal swab, insert the swab into the part with the most saliva in the throat, gently shake the swab on the inner wall of the throat, and take out the swab after sufficient samples are dipped,After collection.
Put the swab containing the sample into the buffer of the Extraction tube , squeeze the sample fully with your fingers for one minute, so that the sample is fully dissolved in the diluent. After the swab is taken out as far as possible, the liquid in the plastic tube is taken as the sample to be tested, and the upper cover of the plastic test tube shall be covered, and the test shall be carried out immediately. If it is not detected for a long time, it needs 2℃~8℃ of refrigeration, the storage time of samples shall not exceed 3 days.
Nasal sampling |
nasopharyngeal sampling |
Oropharyngeal sampling |
 |
 |
 |
[Test procedure]
1. Before testing, test cassette and sample should be restored to room temperature .2. Open the aluminum foil package, take out the test cassette and place it on the horizontal table.
3. Reverse the plastic tube containing the treated sample and add 3 drops (about 90ul) into the sample hole(s) of the test kit. Count for 15 minutes and observe the results. Please note that the result is invalid after 30 minutes.
4. Window “C” is the control line, “T” is the test line.
[Interpretation of the results]
Positive test results: Two purplish red C lines and T lines appear simultaneously in the test area. The results were positive. This indicates that there is COVID 19 in the sample.
Negative test results: The control line(C) is the only visible line in the test area. No COVID 19 antigen was detected. The results do not rule out infection. If symptoms persist, new samples should be taken from patients within 3-5 days and retested.
Invalid test results: If the control line (C) does not appear in the test area, the test result is invalid regardless of whether there are other visible lines in the test area. Retest with a new reagent cassette.
[Product performance index]
1. Inspection of reference materials of the company:
1.1 positive coincidence rate: COVID 19 antigen reference material P1 ~ P8 should be tested positive, with a coincidence rate of 8/8.
1.2 negative coincidence rate: COVID 19 reference material N1 ~ N20 was used for detection. All of them should be negative, with a coincidence rate of 20/20.
1.3 Detection limit: the COVID 19 reference material S1~S6 was used for detection. ,S1 to S4 should be positive, S5 and S6 do not require.
1.4 reproducibility: COVID 19 antigen reference materials R1 and R2 were used for 10 tests. Results should be positive and the chromaticity was uniform.
2. HOOK effect: COVID 19 antigen positive reference material with the highest concentration was detected in the kit without HOOK effect.
3. there was no cross reaction and interference with the potential cross reacting microorganisms listed below.
| substance | strain | concentration | results |
| Human coronavirus | MERS | 5ug/ml | no cross reaction |
| OC43 | 1:20 | no cross reaction | |
| 229E | 1:20 | no cross reaction | |
| HKU1 | 1:20 | no cross reaction | |
|
Influenza B virus |
Yamagata | 1:20 | no interference |
| victoria | 1:20 | no interference | |
| Influenza A virus | H5N1 1:40 | 1:40 | no interference |
| H7N9 1:40 | 1:40 | no interference | |
| H3N2 1:40 | 1:40 | no interference | |
| HIN1 5ug/ml | 5ug/ml | no interference | |
| B-V 5ug/ml | / | 5ug/ml | no interference |
| Measles | / | 1:40 | no interference |
| Rotavirus | / | 1:40 | no interference |
| CMV | / | 1:40 | no interference |
| Chlamydia |
TWAR strain TW-183 |
1:40 | no interference |
| RSV | / | 50ug/ml | no interference |
| Mumps virus | / | 50ug/ml | no interference |
| Adeno-virus | Type 1 | 10ug/ml | no interference |
| Type 2 | 10ug/ml | no interference | |
| Type 3 | 10ug/ml | no interference | |
| Type 4 | 10ug/ml | no interference | |
| Type 5 | 10ug/ml | no interference | |
| Type 7 | 10ug/ml | no interference | |
| Type 55 | 10ug/ml | no interference | |
| Parainfluenza | Type 1 | 5ug/ml | no interference |
| Type 2 | 5ug/ml | no interference | |
| Type 3 | 5ug/ml | no interference | |
| Type 4A | 5ug/ml | no interference | |
| VZ | / | 30ug/ml | no interference |
4. Clinical performance
4.1 nasal specimen samples
Clinical performance of COVID 19 antigen test kit (colloidal gold method) was determined by testing 122 positive and 123 negative specimens for COVID 19 antigen N protein to have a sensitivity of 95.90% [92.38%,99.42%]; specificity of 99.19% [97.60%,10077%]; Total coincidence rate 97.55% [85.18%,109.92%];
| nasal specimen samples | PCR Test Results | Total | ||
| Positive | Negative | |||
| COVID 19 antigen test kit (colloidal gold method) N protein results | Positive | 118 | 1 | 119 |
| Negative | 5 | 122 | 128 | |
| Total | 123 | 123 | 246 | |
| sensitivity | specificity | Total coincidence rate | ||
|
95.94%; [92.44%,99.42%]; |
99.19% [97.60%,10077%]; |
97.56%; [85.18%,109.90%]; |
||
4.2 nasopharyngeal/oropharyngeal specimen samples
Clinical performance of COVID 19 antigen test kit (colloidal gold method) was determined by testing 122 positive and 123 negative specimens for COVID 19 antigen N protein to have a sensitivity of98.36% [96.11%,100.61%]; specificity of 99.19% [97.60%,10077%]; Total coincidence rate 98.78% [86.33%,111.22%];
| nasopharyngeal/oropharyngeal specimen samples | PCR Test Results | Total | ||
| Positive | Negative | |||
| COVID 19 antigen test kit (colloidal gold method) N protein results | Positive | 120 | 1 | 121 |
| Negative | 2 | 122 | 124 | |
| Total | 122 | 123 | 245 | |
| sensitivity | specificity | Total coincidence rate | ||
|
98.36%; [96.11%,100.61%]; |
99.19% [97.60%,10077%]; |
98.78%; [86.33%,111.22%]; |
||
[Precautions]
1. This product is only used for preliminary screening of in vitro diagnosis. Please do not use it as a confirmation reagent. The positive results must be confirmed in combination of clinical symptoms and other tests.
2. Due to methodological or antigen specificity, testing the same sample with reagents from different manufacturers may result in different test
results. Therefore, the results obtained by testing with different reagents should not be directly compared with each other to avoid incorrect medical interpretation. It is recommended that the laboratory indicate the characteristics of the reagents used in the test report issued to the clinician.
3.Reagents should be kept sealed and kept away from moisture. The reagents and the samples stored at low temperature should be equilibrated to room temperature before use.
4.Please follow the general guidelines for biosecurity in microbiology and biomedical laboratories.
5.The wastes including the used reagents and all samples are potentially infected, please dispose of them as infectious medical wastes.
[Symbol]
 |
Consult instructions for use |
 |
Contains sufficient for <n> tests |
 |
In vitro diagnostic medical device |
 |
Caution |
 |
Use by date |
 |
Do not reuse |
 |
Temperature limitation |
 |
Batch code |
 |
Catalogue number |
 |
Keep away from sunlight |
 |
Keep dry |
 |
EU Authorized representative |
 |
Manufacturer |
 |
Prescription only |
 |
Date of manufacture |
 |
Biological risks |
 |
Fragile, handle with care |
 |
Do not use if package is damaged |
 |
This way up |
 Neo-nostics (Suzhou) Bioengineering Co., Ltd.
Neo-nostics (Suzhou) Bioengineering Co., Ltd.
Address: 2F, #3 building, #52 yin gang Road, TaiCang, SuZhou city, Jiang Su, 215434, China
Tel: 086-0512-33022690, 086-0512-33022691
Email: joey.zhou@neo-nostics.com

Osmunda Medical Tchnology Service GmbH
Adresse: Von Oppen-Weg 15, 14476 Potsdam, Germany
DIMDI code: DE/0000047267

The company has been certified by the ISO Quality Control System



Gebrauchsanweisung
[Produktname]
COVID 19 Antigen-Prüfkit (Verfahren mit kolloidalem Gold)[Verpackungsspezifikation]
Prüfkassette (Einzelprüfung-Verpackung):1 Prüfung/Kassette; 5 Prüfungen/Kassette; 10 Prüfungen/Kassette; 15 Prüfungen/Kassette.
[Verwendungszweck]
Dieses Reagenz wird für den qualitativen In-vitro-Test des COVID 19 Antigen N-Proteins in humanen nasalen /Nasopharyngealen und oropharyngealen Proben verwendet. Synthetisches Spinous-Protein und genetische Varianten von UK können nicht nachgewiesen werdenEr wird nur als zusätzlicher Testindikator bei Verdachtsfällen mit negativem Ergebnis des COVID 19-Tests oder in Kombination mit dem 2019-nCov IgG / IgM-Antikörper-Testkit zur Hilfsdiagnose bei Verdachtsfällen verwendet. Er kann nicht als Grundlage für die Bestätigung oder Diagnose einer durch eine COVID 19-Infektion verursachten Lungenentzündung verwendet werden. Ein positives Prüfergebnis erfordert eine weitere Bestätigung, und ein negatives Prüfergebnis kann die Möglichkeit einer Infektion nicht ausschließen.
Das Novel Coronavirus 2019, auch bekannt als COVID 19, ist eine akute Infektionskrankheit der Atemwege. Menschen sind generell empfänglich. Derzeit sind die mit dem COVID 19 infizierten Patienten die Hauptinfektionsquelle; asymptomatische infizierte Personen können ebenfalls eine Infektionsquelle sein. Basierend auf der aktuellen epidemiologischen Untersuchung beträgt die Inkubationszeit 1 bis 14 Tage, meistens 3 bis 7 Tage. Zu den Hauptmanifestationen gehören Fieber, Müdigkeit und trockener Husten. Nasenverstopfung, laufende Nase, Halsschmerzen, Myalgie und Durchfall werden in wenigen Fällen beobachtet.
[Prüfprinzip]
Der COVID 19-Antigen-Prüfkit (kolloidales Goldverfahren) wurde durch kolloidale Gold-Festphasen-Immunochromatographie hergestellt. Ein monoklonaler Antikörper COVID 19 N-Protein wurde auf der Zellulosenitratmembran vorimmobilisiert. Der monoklonale Antikörper gegen das COVID 19 N-Protein wurde mit kolloidalem Gold auf der Glasfasermembran markiert. Der Doppelantikörper-Sandwich-Immunoassay wurde zum Nachweis des N-Proteins des COVID 19 verwendet. Der Kit wurde für den qualitativen In-vitro-Hilfstest von COVID 19-Antigenproben verwendet.Bei der Prüfung werden 3 Tropfen der vorbehandelten Probe (ca. 90 ul) in die Probenöffnung(en) der Prüfkarte gegeben. Wenn das COVID 19-Antigen in der Probe enthalten ist, können COVID 19-Antigene in der Probe kolloidale Goldkomplexe mit kolloidalem Gold markierten Antikörpern bilden. Der Komplex wird zusammen mit dem Prüfstreifen vorwärts bewegt und an der Prüflinienstelle (T) der Nitrocellulosemembran eingefangen. Der überschüssige, nicht konjugierte, mit kolloidalem Gold markierte Antikörper bewegt sich weiter vorwärts zur Position der Qualitätskontrolllinie und wird von dem Festphasen-Antikörper der Qualitätskontrolllinie eingefangen. Auf diese Weise bildet sich an der Position der Prüflinie (T) und der Position der Qualitätskontrolllinie (C) eine mit bloßem Auge sichtbare purpurrote Bande, die ein positives Ergebnis anzeigt. Enthält die Probe kein COVID 19-Antigen oder ist ihr Gehalt niedriger als die Nachweisgrenze, zeigt sie an der Prüflinienposition (T) keine Farbe und an der Qualitätskontrolllinienposition (C) wird nur eine purpurrote Bande angezeigt, was auf ein negatives Ergebnis hindeutet. Wenn an der Qualitätskontrolllinie (C) keine purpurrote Bande zu sehen ist, wird festgestellt, dass das Ergebnis ungültig ist, unabhängig davon, ob an der Position (T) der Prüflinie eine purpurrote Bande zu sehen ist, und es muss erneut bestimmt werden.
[Komponenten]
| 1. Prüfkassette | 2. Tupfer |
| 3. Extraktionsröhrchen | 4. Pufferröhrchen (600 ul/Flasche) |
| 5. Gebrauchsanweisung |
[Lagerbedingungen und Haltbarkeit]
Die Originalverpackung sollte an einem dunklen und trockenen Ort bei 2 °C~30 °C gelagert und nicht eingefroren werden. Die Haltbarkeit des Produkts beträgt 24 Monate. Das Reagenz sollte so schnell wie möglich innerhalb von 1 Stunde nach Öffnen des Alufolienbeutels verwendet werden.Herstellungsdatum: siehe Produktetikett.
Verfallsdatum: siehe Produktetikett.
[Probenentnahme und -verarbeitung]
Bitte verwenden Sie für die Probennahme den in diesem Produkt enthaltenen Tupfer. Drehen Sie vor der Entnahme von nasalem/nasopharyngealem/oropharyngealem Sekret das im Testkit enthaltene Pufferröhrchen, geben Sie den Puffertropfen in das Entnahmeröhrchen und beginnen Sie dann mit der Probenahme.Hinweis: Führen Sie beim Sammeln des Nasensekrets den Tupfer vorsichtig in das 2,5-3 cm entfernte Nasenloch ein und drehen Sie den Tupfer mehrmals kreisförmig an der Innenwand des Nasenlochs, um sicherzustellen, dass so viele Zellen und Schleim wie möglich gesammelt werden. Wiederholen Sie den gleichen Vorgang mit dem gleichen Tupfer im anderen Nasenloch.
Beim Sammeln des nasopharyngealen Sekrets führen Sie den Tupfer vorsichtig in den am meisten sezernierenden Teil des Nasenlochs ein und schieben ihn in Richtung Nasenhöhle bis zum Nasenmuschelknochen. Drehen Sie den Tupfer mehrmals kreisförmig an der Innenwand des Nasenlochs, um sicherzustellen, dass so viele Zellen und Schleim wie möglich gesammelt werden.
Bei der Entnahme des oropharyngealen Tupfers führen Sie den Tupfer in den Teil des Rachens ein, in dem sich am meisten Speichel befindet, schütteln Sie den Tupfer vorsichtig an der Innenwand des Rachens und nehmen Sie den Tupfer heraus, nachdem genügend Proben eingetaucht wurden,Nach der Entnahme.
Legen Sie den Tupfer mit der Probe in den Puffer des Extraktionsröhrchens, drücken Sie die Probe eine Minute lang mit den Fingern vollständig zusammen, so dass die Probe vollständig im Verdünnungsmittel gelöst ist. Nachdem der Tupfer so weit wie möglich herausgezogen wurde, wird die Flüssigkeit im Plastikröhrchen als zu prüfende Probe entnommen, und der obere Deckel des Plastikröhrchens wird geschlossen, und die Prüfung wird sofort durchgeführt. Wenn es für eine lange Zeit nicht benutzt wird, muss es auf 2 ℃~8 ℃ gekühlt werden, die Lagerzeit der Proben sollte 3 Tage nicht überschreiten.
| Nasale Probenahme | Nasopharyngeale Probennahme | Oropharyngeale Probennahme |
 |
 |
 |
[Testverfahren]
1. Vor der Prüfung sollten die Prüfkassette und die Probe auf Raumtemperatur gebracht werden.2. Öffnen Sie die Aluminiumfolienverpackung, nehmen Sie die Prüfkassette heraus und legen Sie sie auf den horizontalen Tisch.
3. Drehen Sie das Kunststoffröhrchen mit der behandelten Probe um und geben Sie 3 Tropfen (ca. 90 ul) in die Probenöffnung(en) der Prüfkassette. Warten Sie 15 Minuten und beobachten Sie die Ergebnisse. Bitte beachten Sie, dass das Ergebnis nach 30 Minuten ungültig ist.
4. Das Fenster “C” ist die Kontrolllinie, “T” ist die Prüflinie.
[Interpretation der Ergebnisse]
Positives Prüfergebnis: Zwei purpurrote C-Linien und T-Linien erscheinen gleichzeitig im Prüfbereich. Die Ergebnisse waren positiv. Dies zeigt an, dass sich COVID 19 in der Probe befindet.Negative Prüfergebnisse: Die Kontrolllinie (C) ist die einzige sichtbare Linie im Prüfbereich. Es wurde kein COVID 19-Antigen nachgewiesen. Das Ergebnis schließt eine Infektion nicht aus. Bei fortbestehenden Symptomen sollten dem Patienten innerhalb von 3-5 Tagen neue Proben entnommen und erneut geprüft werden.
Ungültige Prüfergebnisse: Wenn die Kontrolllinie (C) im Prüfbereich nicht erscheint, ist das Prüfergebnis ungültig, unabhängig davon, ob andere Linien im Prüfbereich sichtbar sind. Wiederholen Sie die Prüfung mit einer neuen Reagenzienkassette.
[Produktleistungsindex]
1. Überprüfung der Referenzmaterialien des Unternehmens:1.1 Positive Koinzidenzrate: COVID 19 Antigen-Referenzmaterial P1 ~ P8 sollte positiv getestet werden, mit einer Koinzidenzrate von 8/8.
1.2 Negative Koinzidenzrate: COVID 19 Referenzmaterial N1 ~ N20 wurde zum Nachweis verwendet. Sie sollten alle negativ sein, mit einer Koinzidenzrate von 20/20.
1.3 Nachweisgrenze: Für den Nachweis wurde das COVID 19 Referenzmaterial S1~S6 verwendet., S1 bis S4 sollten positiv sein, S5 und S6 sind nicht erforderlich.
1.4 Reproduzierbarkeit: Die COVID 19 Antigen-Referenzmaterialien R1 und R2 wurden für 10 Tests verwendet. Die Ergebnisse sollten positiv sein und die Chromatizität war einheitlich.
2. HAKEN-Effekt: Das COVID 19 Antigen positive Referenzmaterial mit der höchsten Konzentration wurde im Kit ohne HAKEN-Effekt nachgewiesen.
3. Es gab keine Kreuzreaktion und Interferenz mit den unten aufgeführten potenziell kreuzreagierenden Mikroorganismen.
| Substanz | Stamm | Konzentration | Ergebnisse |
| Humanes Coronavirus | MERS | 5ug/ml | keine Kreuzreaktion |
| OC43 | 1:20 | keine Kreuzreaktion | |
| 229E | 1:20 | keine Kreuzreaktion | |
| HKU1 | 1:20 | keine Kreuzreaktion | |
|
Influenza-B-Virus |
Yamagata | 1:20 | keine Interferenz |
| victoria | 1:20 | keine Interferenz | |
| Influenza-A-Virus | H5N1 1:40 | 1:40 | keine Interferenz |
| H7N9 1:40 | 1:40 | keine Interferenz | |
| H3N2 1:40 | 1:40 | keine Interferenz | |
| HIN1 5 ug/ml | 5ug/ml | keine Interferenz | |
| B-V 5 ug/ml | / | 5ug/ml | keine Interferenz |
| Masern | / | 1:40 | keine Interferenz |
| Rotavirus | / | 1:40 | keine Interferenz |
| CMV | / | 1:40 | keine Interferenz |
| Chlamydia |
TWAR-Stamm TW-183 |
1:40 | keine Interferenz |
| RSV | / | 50ug/ml | keine Interferenz |
| Mumps-Virus | / | 50ug/ml | keine Interferenz |
| Adeno-Virus | Typ 1 | 10ug/ml | keine Interferenz |
| Typ 2 | 10ug/ml | keine Interferenz | |
| Typ 3 | 10ug/ml | keine Interferenz | |
| Typ 4 | 10ug/ml | keine Interferenz | |
| Typ 5 | 10ug/ml | keine Interferenz | |
| Typ 7 | 10ug/ml | keine Interferenz | |
| Typ 55 | 10ug/ml | keine Interferenz | |
| Parainfluenza | Typ 1 | 5ug/ml | keine Interferenz |
| Typ 2 | 5ug/ml | keine Interferenz | |
| Typ 3 | 5ug/ml | keine Interferenz | |
| Typ 4A | 5ug/ml | keine Interferenz | |
| VZ | / | 30ug/ml | keine Interferenz |
4. Klinische Leistung
4.1 Nasale proben
Die klinische Leistung des COVID 19-Antigen-Testkits (kolloidales Goldverfahren) wurde durch Testen von 122 positiven und 123 negativen Proben auf das COVID 19-Antigen N-Protein bestimmt und ergab eine Sensitivität von 95,90% [92,38%, 99,42%]; Spezifität von 99,19% [97,60%, 100,77%]; Gesamtkoinzidenzrate 97,55% [85,18%, 109,92%];
| Nasale proben | PCR-Prüfergebnisse | Gesamt | ||
| Positiv | Negativ | |||
| COVID 19 Antigen-Prüfkit (kolloidales Goldverfahren) N-Protein-Ergebnisse | Positiv | 118 | 1 | 119 |
| Negativ | 5 | 122 | 128 | |
| Gesamt | 123 | 123 | 246 | |
| Sensitivität | Spezifität | Gesamt-Koinzidenzrate | ||
|
95,94%; [92,44%,99,42%]; |
99,19% [97,60%,100,77%]; |
97,56%; [85,18%,109,90%]; |
||
Die klinische Leistung des COVID 19-Antigen-Testkits (Methode mit kolloidalem Gold) wurde durch Testen von 122 positiven und 123 negativen Proben auf das COVID 19-Antigen N-Protein mit einer Sensitivität von 98,36% [96,11%,100,61%]; Spezifität von 99,19% [97,60%,100,77%]; Gesamtkoinzidenzrate 98,78% [86,33%,111,22%] bestimmt;
| Nasopharyngeale/oropharyngeale Proben | PCR-Prüfergebnisse | Gesamt | ||
| Positiv | Negativ | |||
| COVID 19 Antigen-Prüfkit (kolloidales Goldverfahren) N-Protein-Ergebnisse | Positiv | 120 | 1 | 121 |
| Negativ | 2 | 122 | 124 | |
| Gesamt | 122 | 123 | 245 | |
| Sensitivität | Spezifität | Gesamt-Koinzidenzrate | ||
|
98,36%; [96,11%,100,61%]; |
99,19% [97,60%,100,77%]; |
98,78%; [86,33%,111,22%]; |
||
[Vorsichtsmaßnahmen]
1. Dieses Produkt wird nur für das vorläufige Screening der In-vitro-Diagnose verwendet. Bitte verwenden Sie es nicht als Bestätigungsreagenz. Die positiven Ergebnisse müssen in Kombination mit klinischen Symptomen und anderen Prüfungen bestätigt werden.2. Aufgrund verfahrenstechnischer oder antigener Spezifität kann die Prüfung der gleichen Probe mit Reagenzien verschiedener Hersteller zu unterschiedlichen Prüfergebnissen führen. Daher sollten die Ergebnisse, die durch die Prüfung mit verschiedenen Reagenzien erhalten wurden, nicht direkt miteinander verglichen werden, um eine falsche medizinische Interpretation zu vermeiden. Es wird empfohlen, dass das Labor die Eigenschaften der verwendeten Reagenzien im Prüfbericht für den Kliniker angibt.
3. Die Reagenzien sollten versiegelt und vor Feuchtigkeit geschützt aufbewahrt werden. Die Reagenzien und die bei niedriger Temperatur gelagerten Proben sollten vor der Verwendung auf Raumtemperatur gebracht werden.
4. Bitte beachten Sie die allgemeinen Richtlinien zur Biosicherheit in mikrobiologischen und biomedizinischen Laboratorien.
5. Die Abfälle einschließlich der verwendeten Reagenzien und alle Proben sind potentiell infektiös, bitte entsorgen Sie sie als infektiösen medizinischen Abfall.
[Symbol]
 |
Gebrauchsanweisung beachten |
 |
Enthält ausreichend für <n> Tests |
 |
In-vitro-Diagnostik-Medizinprodukt |
 |
Vorsicht |
 |
Verfallsdatum |
 |
Nicht wiederverwenden |
 |
Temperaturbegrenzung |
 |
Chargennummer |
 |
Katalognummer |
 |
Von Sonnenlicht fernhalten |
 |
Trocken halten |
 |
EU-Bevollmächtigter |
 |
Hersteller |
 |
Verschreibungspflichtig |
 |
Datum der Herstellung |
 |
Biologische Risiken |
 |
Zerbrechlich, mit Vorsicht behandeln |
 |
Nicht verwenden, wenn die Verpackung beschädigt ist |
 |
Diese Seiten nach oben |
 |
Neo-nostics (Suzhou) Bioengineering Co., Ltd. Adresse: 2F, #3 Gebäude, #52 yin gang Str., TaiCang, SuZhou Stadt, Jiang Su, 215434, China Tel: 086-0512-33022690, 086-0512-33022691 E-Mail: joey.zhou@neo-nostics.com |
|
 |
Osmunda Medical Tchnology Service GmbH Adresse: Von Oppen-Weg 15, 14476 Potsdam, Germany DIMDI code: DE/0000047267 |
|
 |
Das Unternehmen ist nach dem ISO-Qualitätssicherungssystem zertifiziert |

Neo Nostics(苏州)生物工程有限公司成立于2015年,是一家专业从事研究,开发,制造和分销先进医疗器械和体外医疗诊断产品的专业公司。我们的产品通常用于生育力测试,传染病测试,滥用药物测试,酒精唾液测试,尿液分析试剂条,心脏标志物测试,肿瘤标志物测试,食品和安全性测试以及动物疾病测试。我们利用先进的工程技术和强大的设计团队可帮助您利用我们独特的零售品牌。我们有能力以简单易用的格式提供高质量的产品,以提供一致的产品性能,将其转化为您和您的客户的价值。

展览会